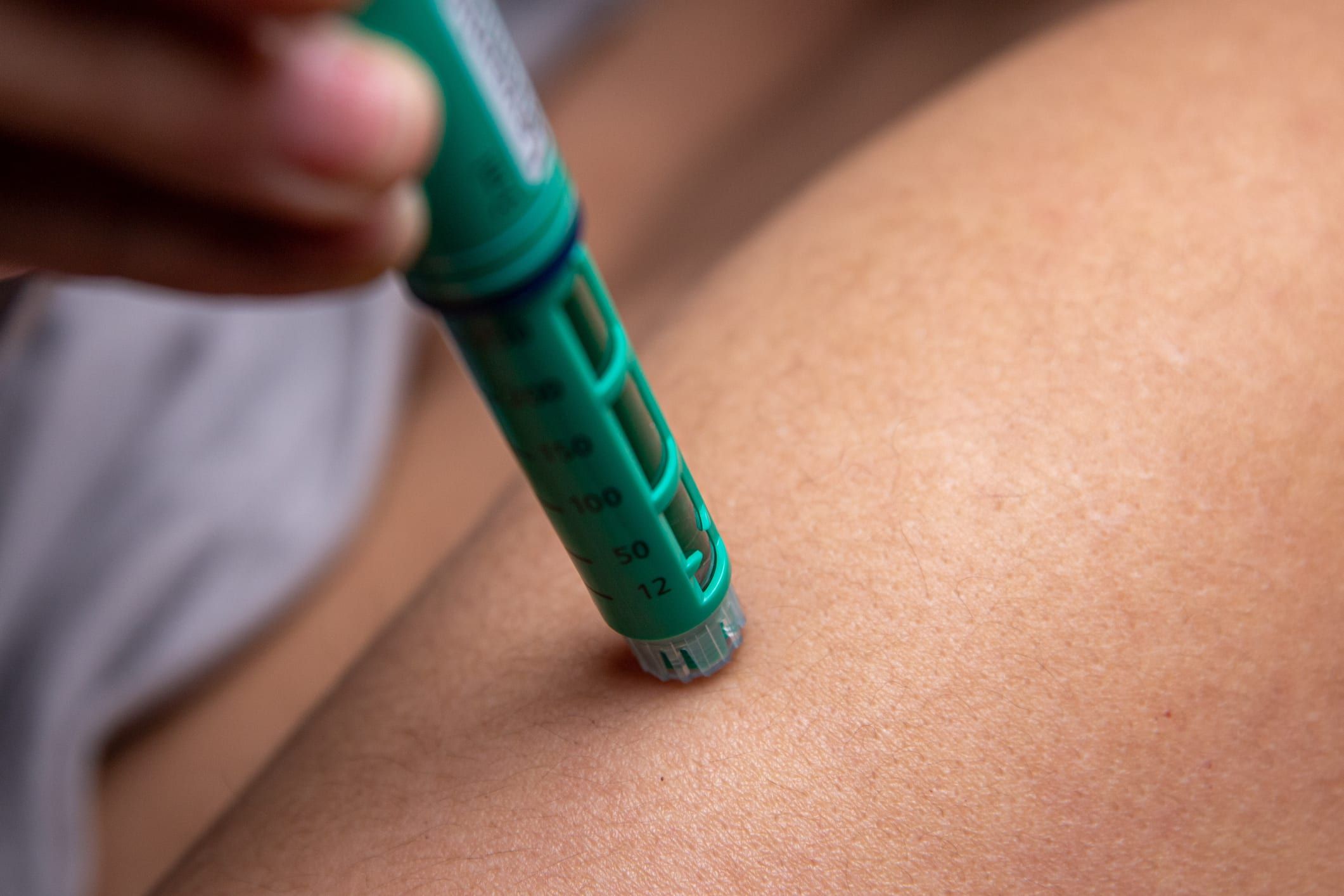
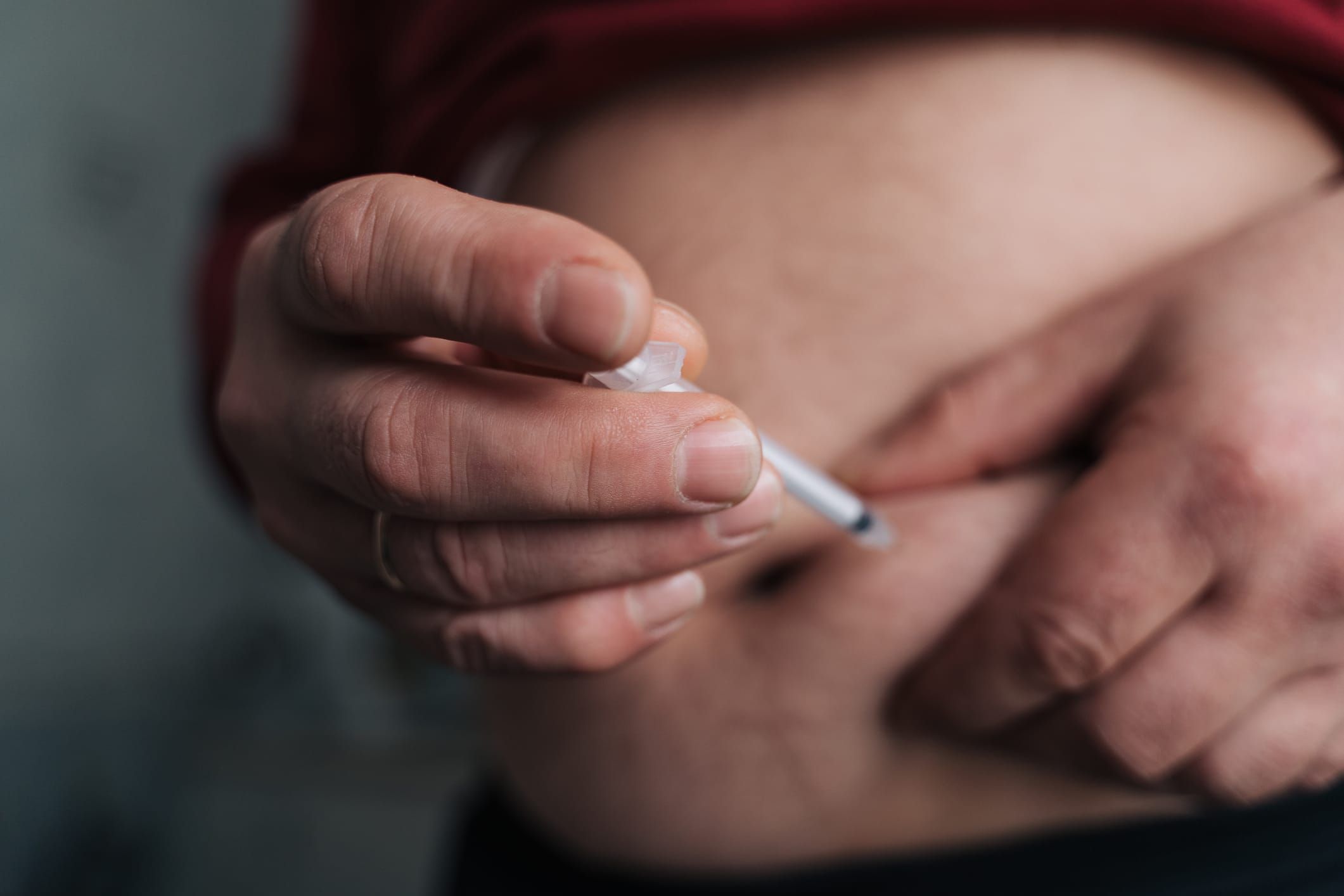
Poison centers have seen an increase in calls of nearly 1,500%.
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement
advertisement